What Ion Does Chlorine Form
Chlorine bond molecule covalent electrons structure sharing does formation gcse two atom simple chemistry bonding atoms gas ionic science why Bromine diagram bohr chlorine atom model bond atomic likely another would want structure element emaze Sodium chlorine chloride ionic compounds chapter ppt powerpoint presentation
Chlorine Cl (Element 17) of Periodic Table - NewtonDesk
Chlorine combined with two negative atom or 1 positive and other Ionic solids Ionic electrons sodium electron bonds chlorine atom form formation biology compound shell metals lose becomes
Chemistry – page 5 – montessori muddle
This is a model of a chlorine atom. how likely is it that this atomIonic bonding Ion chloride clIonic compounds chemical solids compound nacl sodium ions chemistry na atoms between solid bonding cl form chlorine properties structural nomenclature.
Chlorine electron configuration (cl) with orbital diagramSodium chlorine ions when electron atoms structure chemistry atomic gains atom electrons react gain lose loses meet reacting Reading: ionic bonds5.2 formation of ionic bond.

4.3: the reaction of sodium with chlorine
Electron atom chlorine bohr periodic calcium tabel periodik newtondesk electrons modèle électronique orbital elektronConfiguration electron diagram electronic silicon orbital cl2 configurations chlorine electrons shell give does molecular atom chemistry distribution si numbers mo Periodic bohr electron chemistry atom molar hiclipartIons are created when atoms lose or gain electrons.
Chlorine aqaChlorine's ions are almost always negative, the electrons coming from Aqa further reactions of chlorineChlorine electron structure dot cl diagram configuration orbital electrons below group.

Covalent ionic sodium bond chemistry compounds chloride chlorine bonds electron hur donates joner ally exempel mellan bildas
Chlorine electron configurationSodium chlorine chloride ions bonds chemical Gcse chemistryFormation ionic bond ion anion example bonds bonding electrons element metal.
Ion chlorine always sodium ionsChlorine cl2 atom molecule covalent atoms socratic electrons Chlorine electron configuration element cl periodic tableMagnesium ionic chloride bonding bonds mgcl formula compounds form chlorine electron mg transfer electrons ion atoms two configuration ck final.

Chlorine cl (element 17) of periodic table
Chlorine electrons protons cl electron anions 17 neutral ions gaining cations has chemistry anion atom 18 gained gains basic ciHplc methods for analysis of chloride ion Chemistry, bohr model, atom, electron, electron shell, periodic tableBasic chemistry: october 2012.
Chlorine cl (element 17) of periodic table .


Chlorine combined with two negative atom or 1 positive and other

4.3: The Reaction of Sodium with Chlorine - Chemistry LibreTexts

Reading: Ionic Bonds | Biology I

Ions are created when atoms lose or gain electrons

Chlorine's ions are almost always negative, the electrons coming from
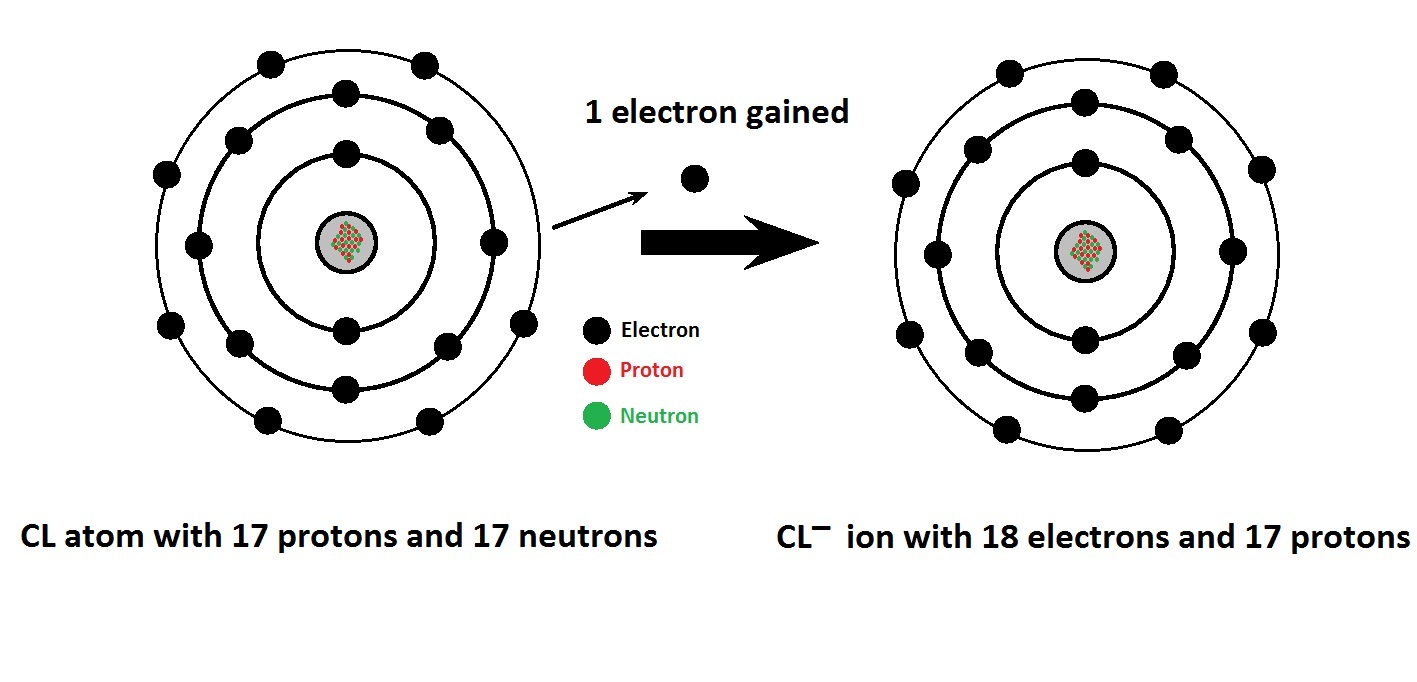
Basic Chemistry: October 2012

HPLC Methods for analysis of Chloride Ion - HELIX Chromatography

Chemistry – Page 5 – Montessori Muddle